Sterile Connections
The infection-free connection of two sterile areas is an essential functionality in many biochemical processes and sometimes also a demanding challenge both for the technology and for the operator of the plant.
This often involves the connection of addition containers for growth media or correction agents or also of harvesting and sampling containers to a bioreactor. If both containers - e.g. the reactor and a media container - are sterile or at least only containing the desired organisms, the challenge is to absolutely prevent foreign germs from getting from one container into the other or from the environment into one of the containers.
What technical possibilities are available to create such a connection between two sterile areas without infection?
The Hypodermic Needle - Diaphragm System
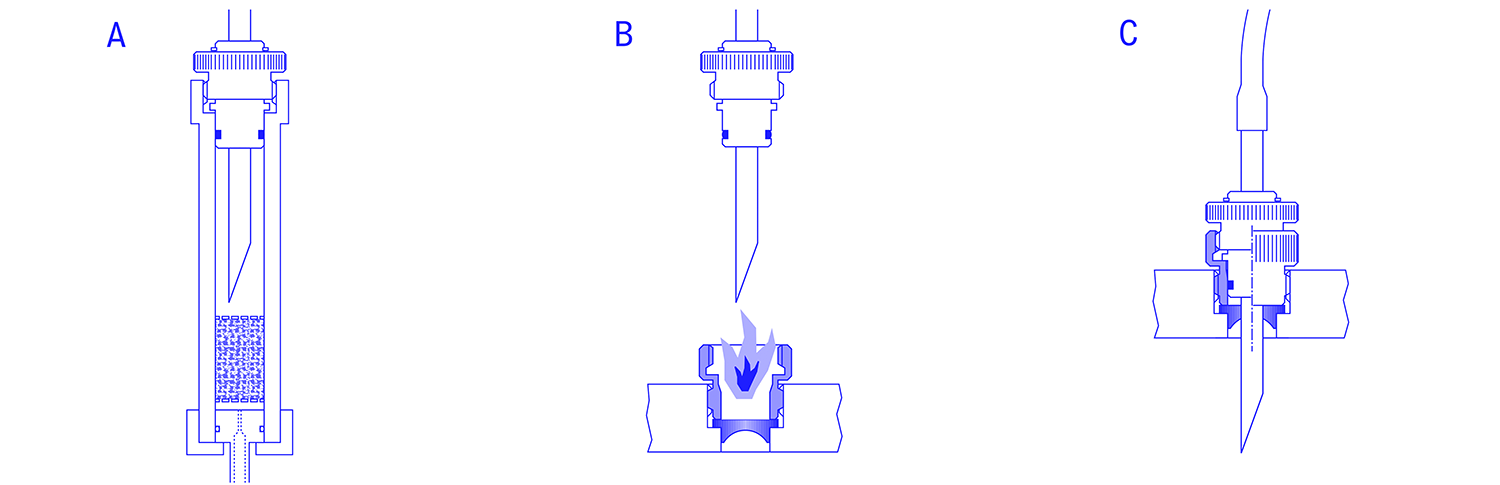
A hypodermic needle is connected to the container via a hose, and this assembly is to be connected to a fermenter, for example, without infection. The needle is protected with a protective sleeve (sterile case) against contamination with foreign germs. The entire unit, needle with protective sleeve, tube and container, is sterilized. On the outer wall of the fermenter, usually on the lid, there is a connection piece compatible with the needle, which is closed against the environment with a suitable polymer diaphragm, protected by a blind plug. The fermenter is also sterile, or possibly already inoculated with a culture.
In order to now connect the two units, vessel and fermenter, without infection, the needle is inserted through the diaphragm and attached to the fermenter nozzle via a thread. The protective sleeve and blind stopper are removed immediately beforehand. In order to further reduce the risk of infection, the piercing process is either carried out under a sterile atmosphere (e.g. a laminar flow system) or ethanol is placed on the diaphragm before the needle is pierced through the diaphragm. If the ethanol is previously ignited, the sterile tube can be removed from the needle directly above the flame and the needle can be pierced through the flame and diaphragm.
The method is characterized by simple, low-maintenance and cost-effective technology. An infection-free connection can thus also be carried out during cultivation. However, the safe, absolutely infection-free production of a connection requires a certain amount of practice and experience on the part of the operating personnel. The method is widely used, especially in research and development.
SteriCap Connection System
The SteriCap valve is connected to the container via a hose, which is to be connected to a fermenter or other vessel type, without infection. The SteriCap valve is sterilized in closed position together with the tube and container in the autoclave. On the outside wall of the fermenter, usually on the lid, there is a connection port compatible with the SteriCap valve. The closed SteriCap is connected to the fermenter via such a connection port. The fermenter is not necessarily sterile at this time and there is no culture in the fermenter.
If the SteriCap is still connected to the fermenter in the closed position, the fermenter is sterilized and thus also the outer surface of the SteriCap valve in the nozzle. Now the valve can be opened, contamination of the fermenter or the connected container with foreign germs is no longer possible.
With the SteriCap, an infection-free connection can be established without the risk of faulty manipulations and with simple hand movements. The SteriCap system meets the requirements of GMP. It is inexpensive, as it does not require its own steam and condensate lines, and takes up little space at the fermenter vessel.
In contrast to the diaphragm-needle system or steam sterilizable sterile crosses, the SteriCap system does not allow any further sterile connections to be made during ongoing cultivation. All necessary sterile compounds must be prepared prior to vessel sterilization, so it is important to plan the fermentation process with foresight.
In contrast to the other systems described, the SteriCap system is not suitable for the connection of containers with inoculum cultures. This is because cultures would have to be positioned next to the fermenter under non-ideal conditions, i.e. hardly stirred, tempered or ventilated, during the entire duration of fermenter sterilization. The inoculum can only be transferred after the fermenter has cooled down sufficiently. For this reason, other systems, such as the injection valve or sterile cross described below, are much more suitable for connecting inoculum vessels. With these systems, the inoculum culture only needs to be placed close to the fermenter for a short time until the liquid can be transferred without infection.
Injection valve
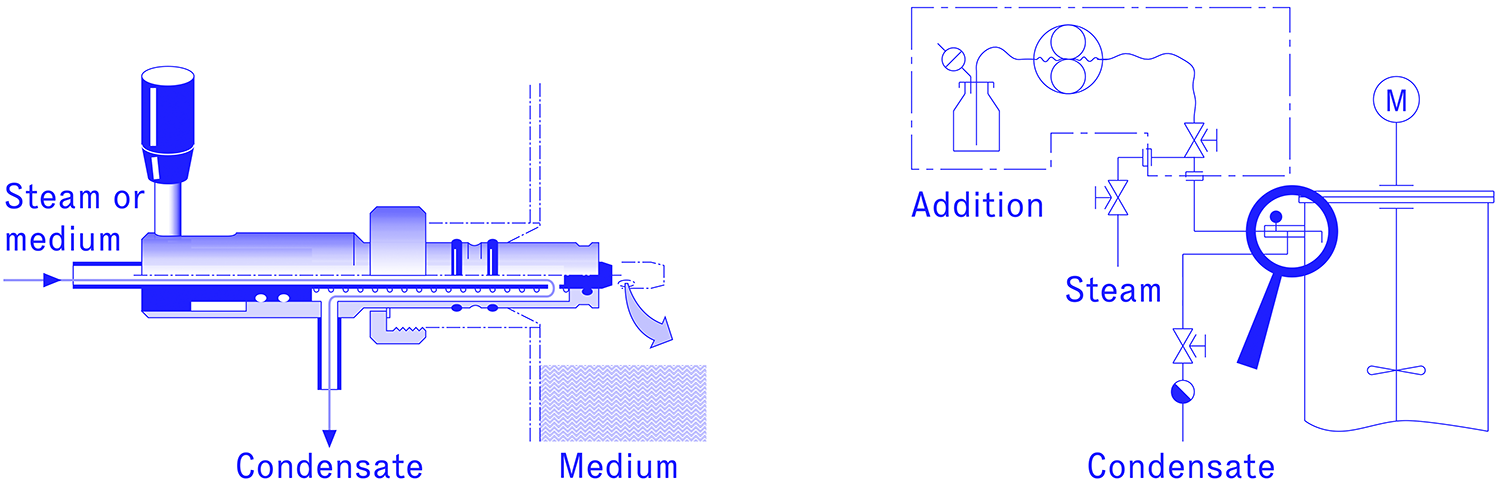
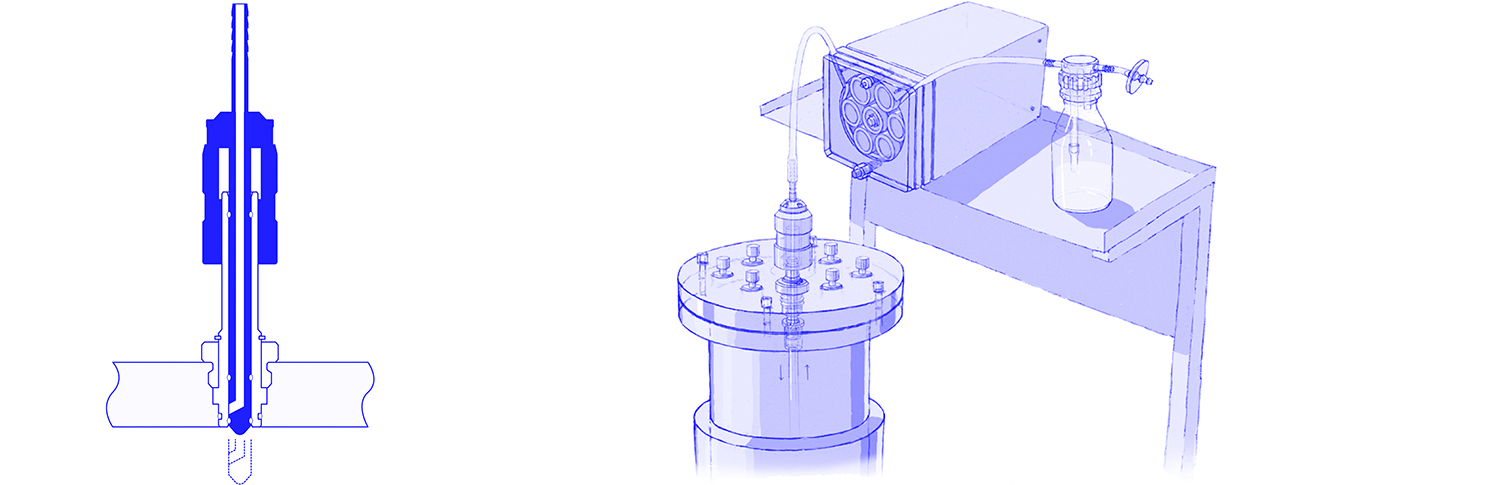
The container, which is to be connected to a fermenter or other vessel without infection, is connected via a hose to a manually operated diaphragm valve, the diaphragm of which forms the sterile boundary. This valve, also known as the "steam sterilizable quick coupling", is sterilized in the closed position together with the tube and container. On the outer wall of the fermenter, usually in the upper, cylindrical part of the boiler, there is a manually operated injection valve which closes directly on the boiler wall, i.e. does not form a dead space against the boiler. The closed diaphragm valve, pre-sterilised together with the hose and container in the autoclave, is connected to this injection valve. The space between the two closed valves is now sterilised with steam via a steam connection on the diaphragm valve and a connection for condensate discharge on the injection valve. Then the two valves can be opened and the liquid transfer can be carried out safely and easily without any risk of infection.
This connection can be re-established and sterilized at any time, even during ongoing cultivation.
The injection valve can also be used to connect larger in-situ sterilizable containers to an in-situ sterilizable fermenter or other vessel via its bottom outlet valve.
With the injection valve in combination with the steam-sterilizable quick coupling, an infection-free connection can be established without the risk of faulty manipulations. It goes without saying that the requirements of GMP are met in full. The connection can also be cleaned in place (CIP-capable) and has the specific advantage that no additional valve for condensate discharge has to be operated when the injection valve is closed due to the absence of any dead space. The system is therefore particularly suitable for cost-effective semi-automatic fermenter systems in which the sterilization of the fermenter vessel is automated, but the automation of additional functions is dispensed with.
Sterile cross

In this solution, too, a container which is to be connected to a fermenter without infection, is connected via a hose to a manually operated steam sterilizable quick coupling (diaphragm valve). But in this case a diaphragm valve is attached to the outer wall of the fermenter via a pipe, which separates the vessel from its surroundings. This diaphragm valve is connected to the quick coupling, which is pre-sterilized together with the hose and container in the autoclave. The space between the two closed valves is sterilized with steam via a steam connection on one diaphragm valve and a connection for condensate discharge on the other diaphragm valve on the fermenter side. The two valves can then be opened and the transfer of the liquid can be carried out safely and easily without any risk of infection. Since steam supply and condensate outlet are also closed or opened by diaphragm valves, there is a group of four valves which can be arranged similarly to a cross - hence the term sterile cross.
As with the injector valve solution, this connection can be re-established and sterilized at any time, even during ongoing cultivation. It is also easy, safe, infection-free and GMP-compliant to be established. Cleaning in place (CIP) is of course possible.
The sterile cross can also be fully automated and, unlike other solutions, it can also be implemented with large line diameters and corresponding valves. Thus media also with high flow rates can be transferred via a sterile cross.
For the safe, infection-free connection of two sterile areas, there are therefore many solutions available which all have their specific advantages and disadvantages depending on the situation. Bioengineering AG is happy to assist in selecting the best solution for the overall system